Titanium Silicalite-1 (TS-1)
Microporous Ti-Si Molecular Sieve, TS-1
Product Detail
CAS No: 13463-67-7 (Titanium Dioxide) / 7631-86-9 (Silicon Dioxide)
We can provide larger packages i.e., 200g, 500g, 1kg, 10kg, 1000kg or based on your need! Please contact us for further information.
Microporous Ti-Si Molecular Sieve TS-1
Titanium Silicalite-1, TS-1, was the first framework-substituted redox molecular sieve to be identified. Redox molecular sieves offer a significant advantage over standard heterogenous catalysts; a redox sieve makes it possible to get a significantly higher selectivity to primary oxidation products. TS-1 has unique properties as a heterogenous catalyst when H2O2 is used as the oxidant and H2O2 is attractive for industrial applications because of its relatively low cost and because it is clean.
ACS Materials carries TS-1 that was prepared using the hydrothermal method. Quantities are available in sizes ranging from 50g to 100kg; contact our customer service representatives to let us know what you need. Our products are trusted by researchers and engineers worldwide for meeting the highest standards of excellence.
1. Preparation Method
Hydrothermal Method
2. Characterizations
|
Form: |
Powder (Type A) Discontinued |
Powder (Type B) |
|
SiO2/TiO2 Molar Ratio: |
≥25 |
≥25 |
|
Particle Size (μm): |
0.3-0.5 |
20-50 |
|
Pore Diameter (nm): |
~0.5 |
~0.5 |
|
BET surface area (m2/g): |
360-420 |
360-420 |
|
Comparative Crystallinity (%): |
>95 |
>95 |
|
Na2O (%): |
<0.1 |
<0.1 |
|
Cation: |
H+ |
--- |
This product is calcined and ready to use directly.

Typical SEM Image of ACS Material TS-1 (Type A)
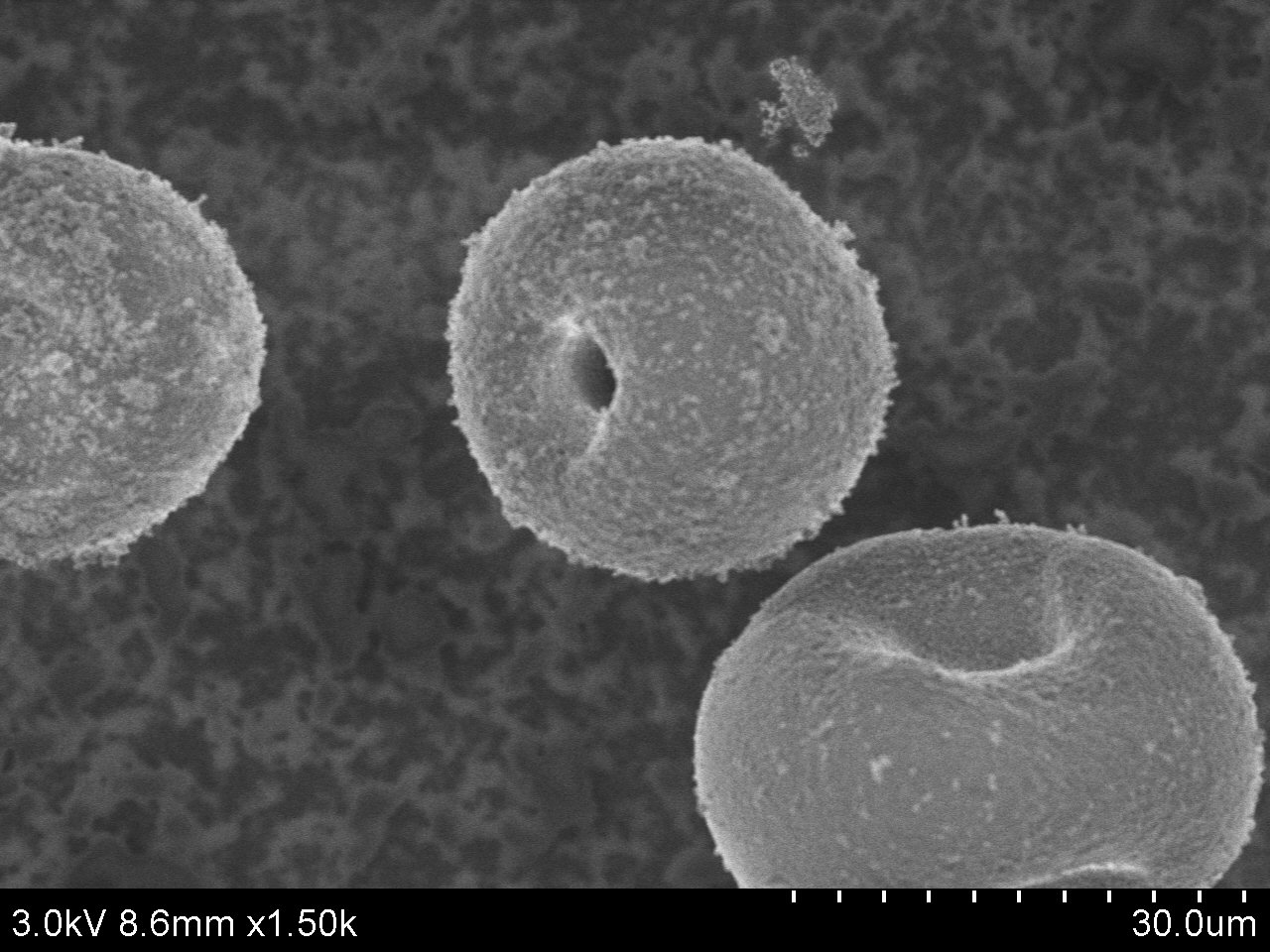
Typical SEM Image of ACS Material TS-1 (Type B)
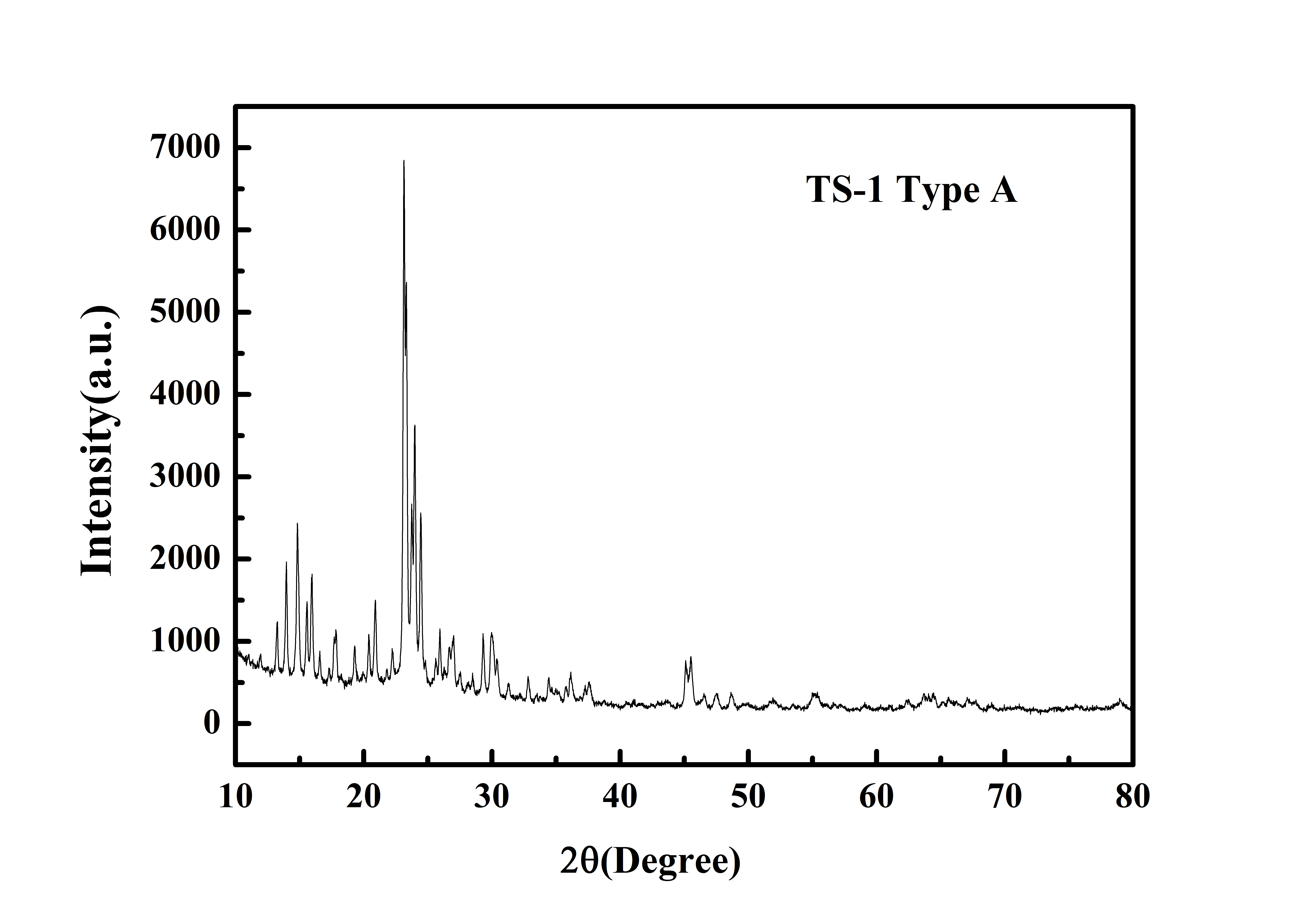
Typical XRD Analysis of ACS Material TS-1 (Type A)
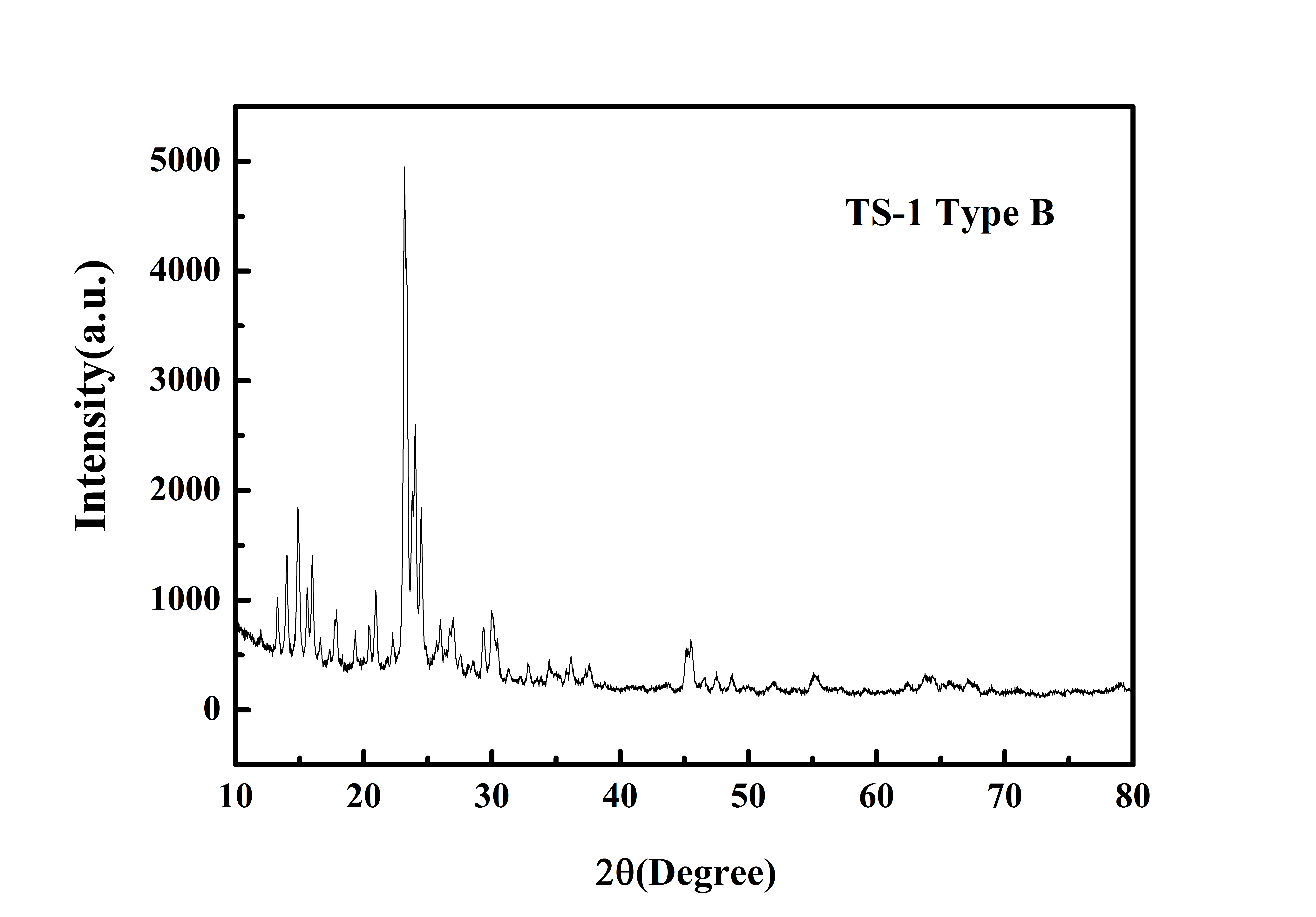
Typical XRD Analysis of ACS Material TS-1 (Type B)
3. Application Fields
Titano silicalite TS-1 has attracted attention due to its excellent catalytic properties in partial oxidation of many organic compounds‚ including:
1) Alkene epoxidation
2) Cyclohexanone ammoxidation
3) Alcohol oxidation
4) Oxidation of saturated hydrocarbon
5) Hydroxylation of aromatic hydrocarbons
Disclaimer: ACS Material LLC believes that the information on our website is accurate and represents the best and most current information available to us. ACS Material makes no representations or warranties either express or implied, regarding the suitability of the material for any purpose or the accuracy of the information listed here. Accordingly, ACS Material will not be responsible for damages resulting from use of or reliance upon this information.
FAQ
1. What is the source of H+ in the Type A of TS-1?
H+ comes from an organic amine contained in the raw material.
2. Is the pH of TS-1 neutral?
Yes, TS-1 is neutral.
3. Is the Powder (Type A) form of the Titanium Silicate-1 neutral or is it acidic?
The Titanium Silacate-1 powder (Type A) form is weakly acidic.
Research Citations of ACS Material Products
- Mukherjee, Mitrajit. Process of Making Olefins or Alkylate by Reaction of Methanol and/or DME or by Reaction of Methanol and/or DME and Butane.
- Rodenas, Y., R. Mariscal, J. L. G. Fierro, D. Martín Alonso, J. A. Dumesic, and M. López Granados. "Improving the production of maleic acid from biomass: TS-1 catalysed aqueous phase oxidation of furfural in the presence of γ-valerolactone." Green Chemistry 20, no. 12 (2018): 2845-2856.
- Rodenas, Y., J. L. G. Fierro, R. Mariscal, M. Retuerto, and M. López Granados. "Post-synthesis Treatment of TS-1 with TPAOH: Effect of Hydrophobicity on the Liquid-Phase Oxidation of Furfural to Maleic Acid." Topics in Catalysis (2019): 1-10.
- Bregante, Daniel T., Alayna M. Johnson, Ami Y. Patel, E. Zeynep Ayla, Michael J. Cordon, Brandon C. Bukowski, Jeffrey Greeley, Rajamani Gounder, and David W. Flaherty. "Cooperative Effects between Hydrophilic Pores and Solvents: Catalytic Consequences of Hydrogen Bonding on Alkene Epoxidation in Zeolites." Journal of the American Chemical Society (2019).
